Biopuremax & Regulatory Authorities:
Biopuremax technologies ESR™ and HOD™ are included in the PW/WFI water production ISO 22519 standard. The systems are also included in the ISPE Pharmaceutical Engineering Guide Volume 4, Water and steam. In addition it is one of the only pretreatment systems that can produce WFI pharmaceutical grade water reliably without distillation due to the very low bacteria levels in the pretreatment.
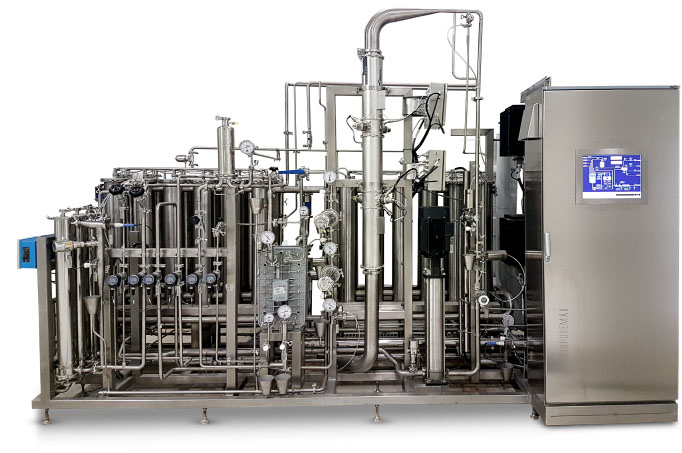
The Biopuremax purified water systems comply with all the strictest requirements and standards of the industry for pharmaceutical water systems.
Industry standards:
- EU cGMP
- FDA cGMP
- United States Pharmacopeia, USP PW
- European Pharmacopoeia, PW
- ASME BPE-2016
- 21 CFR part 11 / Annex 11
- GAMP 5
- 21 CFR part 210: cGMP in manufacturing, processing, packing and holding of drugs
- 21 CFR part 211: cGMP for finished pharmaceuticals
- FDA industry guideline: cGMP Guideline of inspection of preparation and drug manufacturer
- EU-GMP-Guideline Part 1, Annexes 1, 15 & 17
- Medicines and Healthcare products Regulatory Agency (MHRA)
- ISPE Pharmaceutical Engineering Guide, Volume 4: Water and Steam Systems
- ISPE Pharmaceutical Engineering Guide, Volume 5: Commissioning and Qualification
- TEMA
Quality standards:
- CE Mark
- ISO 13475
- ISO 9001
- ISO 22519
 German
German English
English Brazil
Brazil
